Atoms are composed of which of the following subatomic particles?
1. Atoms are composed of which of the following subatomic particles?
a.Photons, neutrinos, and electrons
Elevate Your Writing with Our Free Writing Tools!
Did you know that we provide a free essay and speech generator, plagiarism checker, summarizer, paraphraser, and other writing tools for free?
Access Free Writing Toolsb.Neutrons and electrons
c.Protons, neutrons, and electrons
d.Protons and electrons
e. Protons and neutrons
Answer: protons, neutrons, and electrons
Question 2. collection of 1.25×1019 electrons has the charge ?
What is An Atom?
An atom is a tiny particle that uniquely defines a chemical element. The atom is the smallest particle in which a chemical element can exist. The atom has a positively charged center with protons and neutrons, surrounded by negatively charged electrons.
So, atoms are the basic building blocks of matter. They account for anything that has mass and occupies space. Sometimes, atoms from different elements interact and form compounds by sharing their electrons.
This post will define the atom’s nature, structure, and history.
What are Protons, Neutrons, and Electrons?
Inside an atom are protons, neutrons, and electrons. Each of these subatomic particles has its special responsibility in the stability of an atom.
Electrons are the light negatively charged subatomic particles that occupy the space surrounding the nucleus. Each electron has a given electrical charge of -1.
A neutron is a subatomic particle found inside the nucleus of every atom. Only simple hydrogen lacks the neutron in its nucleus. As the name suggests, the neutron has a neutral electric value. Usually, these particles are dense in mass and thus occupy the center of the atom. The rest mass of a neutron, mn, is about 1.675 x 10-27 kg.
Protons are dense, positively charged subatomic particles. Due to their density, protons occupy the center of the atom together with the neutrons. A proton has a rest mass mp of 1.673 x 10-27 kg. The number of protons in an atom is unique for every element. So, the number of protons in an atom is denoted as its atomic number and determines a chemical element’s place in the periodic table.
The rest mass of these subatomic particles is low but increases tremendously during extreme speed, like in a linear accelerator or cyclotron.
The Structure of an Atom
While electrons occupy the outer spaces of the atom, the protons and neutrons join to form quarks. The quarks consist of the protons and neutrons of an atom, and hence its nucleus. Strong nuclear force holds the nucleus of an atom together.
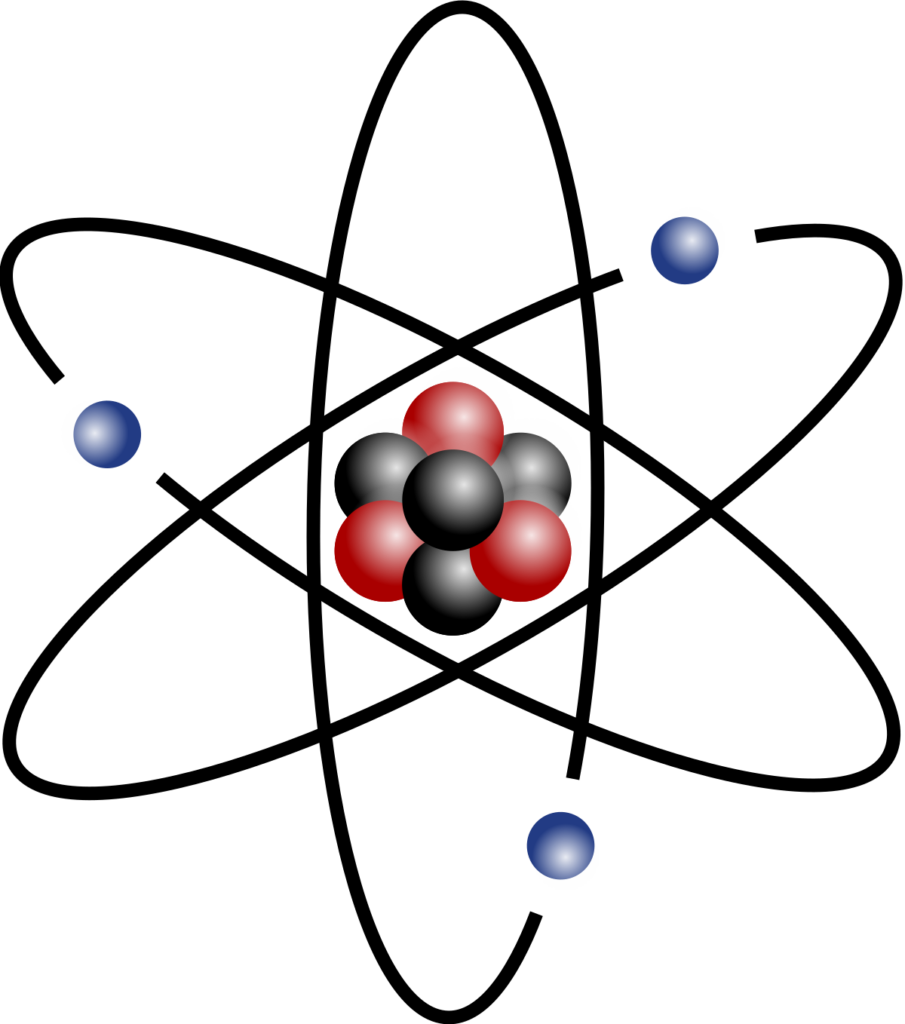
Atomic mass or weight is the atom’s total mass, including all electrons, neutrons, and protons, and is measured in atomic mass units. However, electrons contribute a negligible portion of that mass. So, atomic mass is typically the number of protons and neutrons in the nucleus of an atom.
Some elements have atoms with a varying number of neutrons and hence vary in atomic mass. Atoms of the same element with different atomic weights are known as isotopes.
Electrons, regardless, play an important role in chemical reactions. Atoms from different elements share electrons to form molecules and other compounds.
Protons and electrons have equal but opposite charges. Atoms are usually neutrally charged because they have an equal number of protons and electrons.
An atom with extra electrons is a negatively charged ion, also known as an anion. Likewise, an atom with an electron deficit is a positively charged ion, also known as a cation.
History of The Atom
Proponents of the Big Bang Theory claim that the history of atoms began 13.7 billion years ago. According to the European Council for Nuclear Research, atoms emerged in the first few minutes of the Big Bang.
The universe started cooling and expanding, creating an optimum condition for the emergence of electrons and quarks. In a few millionths of a second after the formation of quarks, they aggregated into neutrons and protons.
The quarks then formed the nuclei of atoms. The positively charged atomic nuclei would then attract a proportional number of electrons to spin around it. And that’s how quarks and electrons formed atoms of different elements.
More questions on Liberty BUSI411

Special offer! Get 20% discount on your first order. Promo code: SAVE20